Por favor, use este identificador para citar o enlazar a este item:
http://hdl.handle.net/10261/111393COMPARTIR / EXPORTAR:
 SHARE SHARE
 CORE
BASE CORE
BASE
|
|
| Visualizar otros formatos: MARC | Dublin Core | RDF | ORE | MODS | METS | DIDL | DATACITE | |

| Campo DC | Valor | Lengua/Idioma |
|---|---|---|
| dc.contributor.author | Sicilia, Violeta | - |
| dc.contributor.author | Borja, Pilar | - |
| dc.contributor.author | Casas, José M. | - |
| dc.contributor.author | Fuertes, Sara | - |
| dc.contributor.author | Martín, Antonio | - |
| dc.date.accessioned | 2015-02-26T12:27:14Z | - |
| dc.date.available | 2015-02-26T12:27:14Z | - |
| dc.date.issued | 2013 | - |
| dc.identifier | doi: 10.1016/j.jorganchem.2013.01.027 | - |
| dc.identifier | issn: 0022-328X | - |
| dc.identifier.citation | Journal of Organometallic Chemistry 731: 10-17 (2013) | - |
| dc.identifier.uri | http://hdl.handle.net/10261/111393 | - |
| dc.description.abstract | The half-lantern compound [{Pt(bzq)(μ-C7H4NOS- κN,S)}2] (2) (bzq = benzo[h]quinoline, C7H 4NOS = 2-mercaptobenzoxazolate) was prepared selectively by reaction of equimolar amounts of potassium 2-mercaptobenzoxazolate (KC7H 4NOS, 1) and [Pt(bzq)(NCMe)2]ClO4. Complex 2 undergoes two-electron oxidation by reaction with halogens X2 (X 2: Cl2, Br2 or I2) to give the corresponding dihalodiplatinum (III) complexes [{Pt(bzq)(μ-C 7H4NOS-κN,S)X}2] (X = Cl 3, Br 4, I 5). The X-ray structures of 3-5 confirm the retention of the half-lantern structure and the shortening of the Pt-Pt distance (Pt-Pt = 2.6383 (3) Å 3, =2.6671 (9) Å 4, 2.6810 (5) Å 5) with respect to that in 2 (2. 9726 (8) Å) because of the Pt-Pt bond formation. The trend of increasing Pt-Pt distances is in accordance with the trans-influence of the axial ligand (Cl < Br < I). DFT calculations on complex 2 in CH2Cl2 indicate the lowest energy absorption band to be mainly due to singlet metal-metal-to-ligand charge transfer (1MMLCT) [dσ *(Pt-Pt) → π*(bzq)] really affected by the π⋯π contacts in the complex, with just a little ligand-to-ligand charge transfer/intraligand (L'LCT/IL) character. The triplet 3MMLCT excited state seems to be the responsible of the structureless red emission of 2 in the solid state and in solution at room temperature. The quantum yield (90%) of this red emission in toluene solution at room temperature is really high. This fact added to the neutral character and the thermal stability of 2 make it a potential compound to be incorporated as phosphorescent dopant in multi-layer OLEDs. © 2013 Elsevier B.V. All rights reserved. | - |
| dc.description.sponsorship | This work was supported by the Spanish MICINN/FEDER (Project CTQ2008-06669-C02), and the Gobierno de Aragón (Grupo Consolidado: Química Inorgánica y de los Compuestos Organometálicos) and P. B. acknowledge the support of an FPI grant from the MICINN. | - |
| dc.publisher | Elsevier | - |
| dc.relation.isversionof | Postprint | - |
| dc.rights | openAccess | - |
| dc.subject | X-ray study | - |
| dc.subject | Half-lantern compound | - |
| dc.subject | Luminescence | - |
| dc.subject | TD-DFT calculation | - |
| dc.subject | Coordination chemistry | - |
| dc.subject | Platinum | - |
| dc.title | Selective synthesis of new half-lantern benzoquinolate platinum complexes. DFT and photophysical studies on the platinum (II,II) derivative | - |
| dc.type | artículo | - |
| dc.identifier.doi | 10.1016/j.jorganchem.2013.01.027 | - |
| dc.relation.publisherversion | http://dx.doi.org/10.1016/j.jorganchem.2013.01.027 | - |
| dc.embargo.terms | 2015-05-01 | - |
| dc.date.updated | 2015-02-26T12:27:14Z | - |
| dc.description.version | Peer Reviewed | - |
| dc.language.rfc3066 | eng | - |
| dc.contributor.funder | Gobierno de Aragón | - |
| dc.contributor.funder | Ministerio de Ciencia e Innovación (España) | - |
| dc.contributor.funder | European Commission | - |
| dc.relation.csic | Sí | - |
| dc.identifier.funder | http://dx.doi.org/10.13039/501100004837 | es_ES |
| dc.identifier.funder | http://dx.doi.org/10.13039/501100000780 | es_ES |
| dc.identifier.funder | http://dx.doi.org/10.13039/501100010067 | es_ES |
| dc.type.coar | http://purl.org/coar/resource_type/c_6501 | es_ES |
| item.fulltext | With Fulltext | - |
| item.openairecristype | http://purl.org/coar/resource_type/c_18cf | - |
| item.cerifentitytype | Publications | - |
| item.grantfulltext | open | - |
| item.openairetype | artículo | - |
| Aparece en las colecciones: | (ISQCH) Artículos | |
Ficheros en este ítem:
| Fichero | Descripción | Tamaño | Formato | |
|---|---|---|---|---|
| Def-Graphical Abstract.pdf | 99,66 kB | Adobe PDF |  Visualizar/Abrir | |
| Def-Highlights.pdf | 104,95 kB | Adobe PDF |  Visualizar/Abrir | |
| Revised-Manuscript.pdf | 478,83 kB | Adobe PDF |  Visualizar/Abrir | |
| Supporting Information.pdf | 489,48 kB | Adobe PDF |  Visualizar/Abrir | |
| SC1.jpg | 104,56 kB | JPEG | 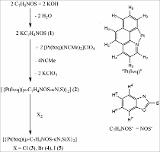 Visualizar/Abrir | |
| FIG1A.jpg | 38,11 kB | JPEG | 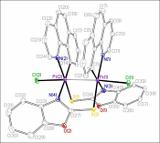 Visualizar/Abrir | |
| FIG1B.jpg | 64,85 kB | JPEG |  Visualizar/Abrir | |
| FIG2.jpg | 48,68 kB | JPEG |  Visualizar/Abrir | |
| Fig.3a.pdf | 351,3 kB | Adobe PDF | 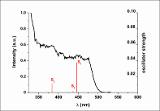 Visualizar/Abrir | |
| fig3b.pdf | 212,35 kB | Adobe PDF |  Visualizar/Abrir | |
| FIG4.jpg | 49,78 kB | JPEG |  Visualizar/Abrir | |
| FIG5.jpg | 51,21 kB | JPEG | 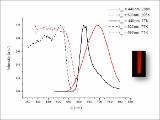 Visualizar/Abrir |
CORE Recommender
SCOPUSTM
Citations
23
checked on 24-abr-2024
WEB OF SCIENCETM
Citations
22
checked on 28-feb-2024
Page view(s)
347
checked on 24-abr-2024
Download(s)
859
checked on 24-abr-2024
Google ScholarTM
Check
Altmetric
Altmetric
NOTA: Los ítems de Digital.CSIC están protegidos por copyright, con todos los derechos reservados, a menos que se indique lo contrario.
